

The majority of traces can be eliminated with careful washing. It is not a threat to the body from the outside.
#Radioactive element definition skin
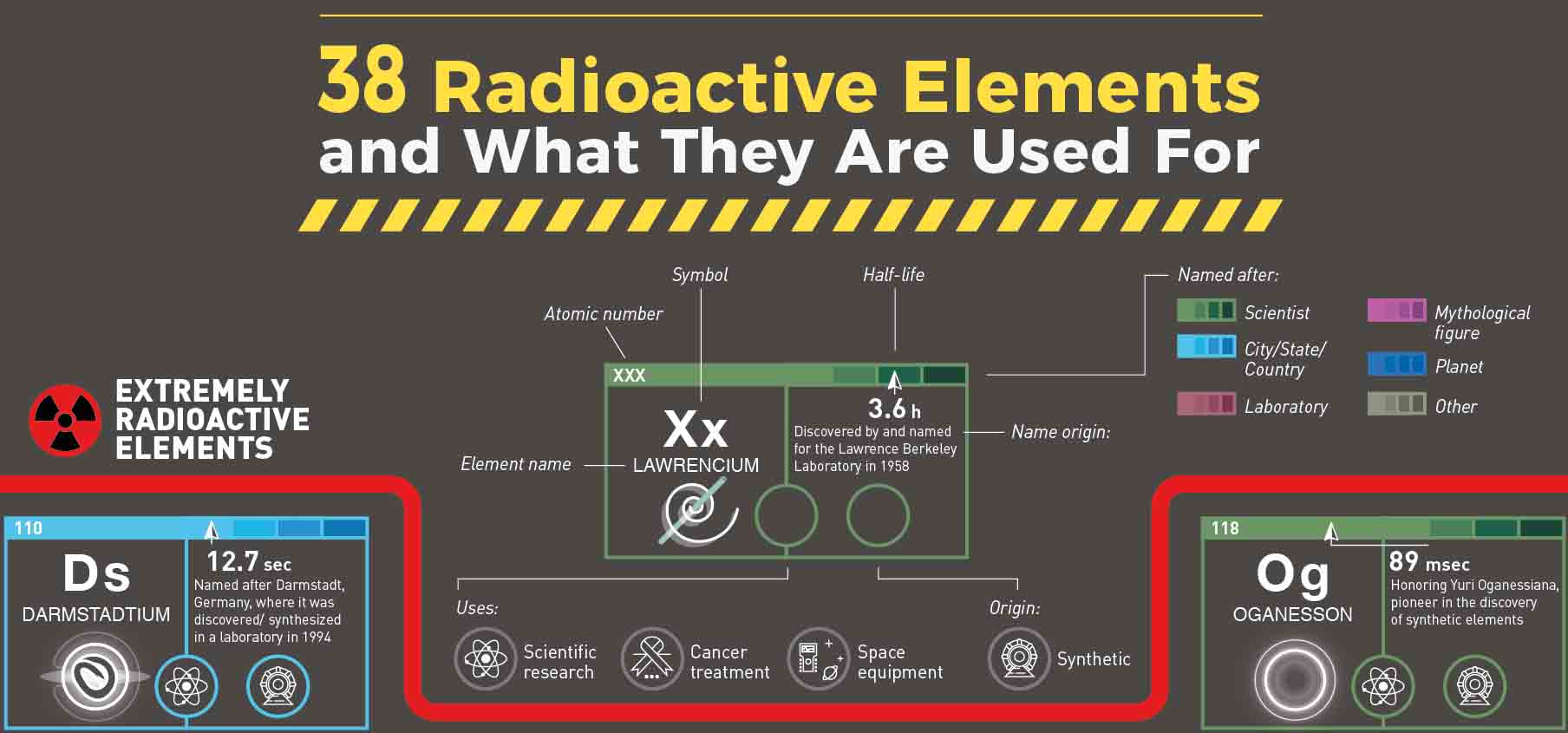
Po-210 and its radiation pass through intact skin and membranes but not intact skin and membranes. Internal contamination can result in organ irradiation, which can cause serious medical symptoms or death. Only when Po-210 enters the body through breathing, eating, or a wound does it become a radiation hazard. It can be made in university or government nuclear reactors, but it takes a lot more expertise. Polonium (Po-210) is a radioactive element that exists in nature at extremely low concentrations. Uranium decays into radium, which then decays into radon over billions of years. Radon is produced by the decay of uranium, a naturally occurring radioactive element found in the Earth's crust. Radon is a radioactive gas that is colourless, odourless, and tasteless. Marie and Pierre Curie are the ones who invented radioactive element radium. Radioactive element Radium is a radioactive metal that can be found in soil, water, rocks, plants, and food at variable levels across Vermont and the entire Earth. Radium and radon are two elements that decay or break down slowly from uranium. Uranium is a radioactive element present in soil, water, rocks, plants, and food. Alpha radiation is one type of this energy. These two elements slowly change form over billions of years, generating decay products like radium and radon. Uranium and thorium, for example, are two radioactive elements that occur naturally in the Earth's crust. When certain radioactive elements decay or break down, they release alpha radiation, which is a type of energy. The radioactive elements listed below are found naturally in the environment. Since then, a score of new elements (with many different isotopes) have been found – mostly by planned nuclear reactions – bringing the total number of elements in the modern Periodic Table to 118. The source of isotopes became clear when James Chadwick (1891-1974) discovered the neutron in 1932 – isotopes have the same atomic number but varied atomic masses. With his notion of isotopes, Frederick Soddy (1877-1956) explained the problem in 1913, proving that some elements may have differing half-lives but same chemical behaviour – hence, they exist in the "same place" (the Greek term "isos topos") in the Periodic Table. However, an issue occurred: plenty of new elements appeared, each with a different half-life, yet many of them had similar, if not identical, chemical properties. Henri-Antoine Becquerel (1852-1908), who discovered radioactivity in 1896, entered a new age of unstable elements. Let us study what a radioactive element is, its history, uses and more details from this article. These are radioactive elements, which means they release energy and break down into new elements over time.

And, while your coffee table or textbook may appear to be quite stable, some elements, particularly those that make up the things in your home, deteriorate over time.
Even though these atoms are far too small to see, everything in an object or organism is ultimately made up of these tiny particles. Everything in our environment is made up of elements, or various types of atoms.


 0 kommentar(er)
0 kommentar(er)
